From Obesity to Alzheimer’s
GLP-1 Drugs and the New Frontier of Brain Inflammation Research
Why in News:
Danish scientist Lotte Bjerre Knudsen, co-inventor of the first long-acting GLP-1 receptor agonist, was recently awarded the Lasker~DeBakey Clinical Medical Research Award (along with collaborators Joel Habener and Svetlana Mojsov) for her decades-long contribution to the development of GLP-1-based drugs that transformed the treatment of obesity and type 2 diabetes. Now, GLP-1 research is entering a new phase, with focus on its potential to reduce brain inflammation, especially in neurodegenerative diseases like Alzheimer’s.
This major breakthrough in medical science is crucial for CLAT 2026 aspirants, especially under CLAT Current Affairs 2026, legal bioethics, and topics on medical innovation, intellectual property rights, and public health. It also connects well with students preparing through the best online coaching for CLAT and other online coaching for CLAT, particularly under interdisciplinary subjects that merge law, science, and ethics.
Introduction:
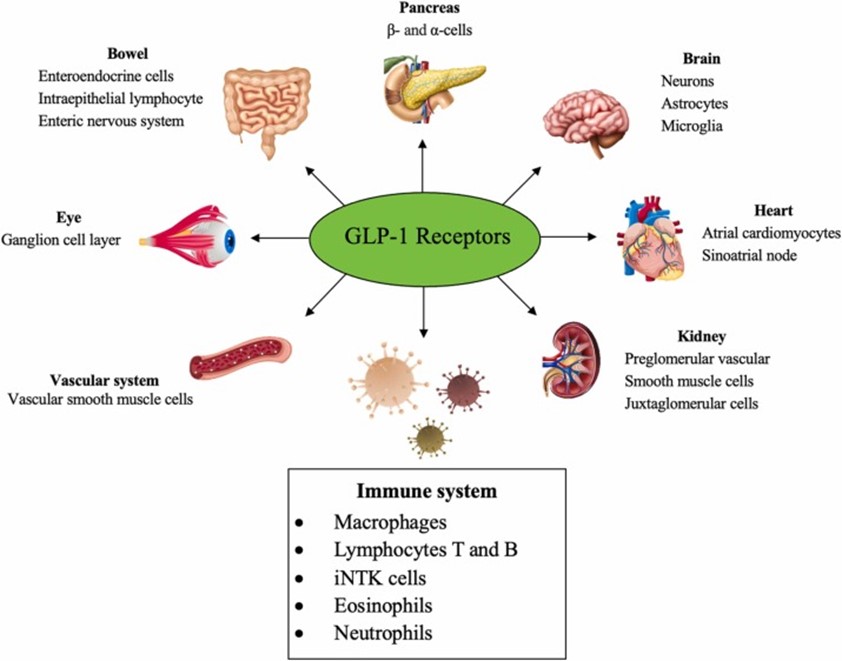
The discovery and development of GLP-1 (Glucagon-like Peptide-1) receptor agonists have significantly changed the treatment landscape for type 2 diabetes and obesity. Now, these drugs are showing promising potential in neuroinflammation management, paving the way for application in treating Alzheimer’s disease. This paradigm shift focuses not just on metabolic control but also on brain-based therapeutic targets.
In a revealing interview with The Indian Express, Lotte Bjerre Knudsen discusses her journey, the science behind GLP-1 analogues like semaglutide and liraglutide, and their next frontier in neurological research. This insight provides CLAT aspirants an exceptional opportunity to understand the intersection of medicine, law, regulation, and innovation.
Point-wise Summary:
- Who is Lotte Bjerre Knudsen?
- Danish scientist awarded for co-developing the first long-acting GLP-1 receptor agonist.
- Her research revolutionized obesity and type 2 diabetes treatment.
- Now exploring GLP-1’s role in Alzheimer’s disease, based on its anti-inflammatory action in the brain.
- What is GLP-1 and What Does It Do?
- GLP-1 (Glucagon-Like Peptide-1) is a natural hormone that:
- Enhances insulin secretion.
- Suppresses glucagon (a hormone that raises blood sugar).
- Slows gastric emptying.
- Reduces appetite and promotes weight loss.
- How Do GLP-1 Drugs Work?
- Two major analogues:
- Semaglutide (e.g., Ozempic, Wegovy).
- Liraglutide (e.g., Victoza, Saxenda).
- Modified to resist degradation and increase half-life (e.g., by adding a fatty acid chain and using enzyme blockers).
- Can last up to 160 hours in the body (vs natural GLP-1 which lasts 2 minutes).
- Transition from Obesity to Alzheimer’s Treatment:
- Researchers are now testing whether GLP-1 drugs can reduce neuroinflammation in Alzheimer’s patients.
- The hypothesis: inflammation in the brain is a key driver of Alzheimer’s.
- GLP-1 may modulate the brain’s immune system and reduce damage caused by inflammation.
- Importance of the “Big Eureka” Moment:
- Success came when scientists extended GLP-1’s action from 2 minutes to 160 hours.
- This allowed the drug to be taken weekly (not daily), enhancing patient compliance.
- Mechanism of Drug Modification:
- GLP-1 is linked to a fatty acid, enabling it to bind to albumin (a protein in blood) for slow release.
- Structural tweaks help avoid enzymatic breakdown.
- The drug mimics natural GLP-1 but remains active for days.
- Clinical Success and Challenges:
- Wegovy and Ozempic have shown dramatic results in reducing weight and diabetes progression.
- Studies now underway to assess impact on heart attack, stroke, and Alzheimer’s symptoms.
- Some experts remain cautious, noting the high cost and limited long-term neurological data.
- The Intellectual and Research Leap:
- Drug development spanned decades, requiring rigorous trials.
- A global collaboration between institutions: Novo Nordisk, Harvard Medical School, Rockefeller University.
- Legal and Policy Implications:
- Patent protections and licensing on GLP-1 analogues have fueled pharma profits but raised concerns about accessibility.
- Ethical debates arise around using obesity medications for cosmetic weight loss.
- Health policy will need to assess cost-benefit and public health affordability for newer GLP-1 applications.
Legal and Ethical Relevance for CLAT 2026:
- Public Health and Right to Medicine:
- Article 21 of the Indian Constitution (Right to Life) includes access to life-saving drugs.
- If GLP-1 proves effective against Alzheimer’s, its accessibility and affordability may become a legal issue.
- Intellectual Property Rights:
- GLP-1 analogues are patent-protected innovations.
- Raises the balance between IP protection vs compulsory licensing for public interest.
- Clinical Trials and Regulation:
- Falls under India’s Drugs and Cosmetics Act, 1940, and New Drugs and Clinical Trials Rules, 2019.
- Research on repurposing GLP-1 for Alzheimer’s must adhere to ethics committee approvals.
- Bioethics and Off-Label Use:
- Using diabetes drugs for neurological conditions raises questions about off-label prescriptions.
- May require stricter regulatory scrutiny and guidelines.
Notes: Explanation of Peculiar Terms
- GLP-1: Glucagon-Like Peptide-1, a hormone regulating insulin and appetite.
- Agonist: A substance that activates a receptor to produce a biological response.
- Neuroinflammation: Inflammation of the nervous tissue, often associated with Alzheimer’s.
- Albumin Binding: A strategy to prolong drug half-life by binding it to blood proteins.
- Off-Label Use: Prescribing a drug for a condition not approved by regulatory agencies.
- Lasker-DeBakey Award: Prestigious recognition for medical research, considered a precursor to the Nobel Prize.
Conclusion:
The evolution of GLP-1 from a metabolic drug to a potential neurological therapy for Alzheimer’s signals a transformative moment in medicine. The journey of Lotte Knudsen and her team underlines the power of sustained innovation, scientific precision, and regulatory foresight. As GLP-1 drugs step into new clinical roles, legal, ethical, and accessibility frameworks will be equally vital.
For CLAT 2026 aspirants, this topic offers cross-disciplinary insight into law, medicine, patent regulation, clinical trials, public health, and technology ethics—making it a likely candidate for comprehension-based passages, legal reasoning scenarios, and essay writing.
This Blog is Powered by CLAT Gurukul — India’s Leading Law Entrance Prep Platform
At CLAT Gurukul, we believe in empowering future legal minds with the right blend of knowledge, strategy, and mentorship. This blog is a reflection of our commitment to quality content that not only helps aspirants stay updated but also sharpens their conceptual clarity.
Why CLAT Gurukul?
- Personalized Mentorship by Top Legal Educators
- Comprehensive Study Materials & Legal Updates
- Daily Practice Sets, Mocks & Performance Tracking
- Result-Oriented Strategy for CLAT, AILET, and CUET
Whether you’re reading this article to deepen your understanding or to stay ahead in your exam prep — you’re already one step closer with CLAT Gurukul by your side.
Join thousands of successful aspirants who trusted CLAT Gurukul and cracked India’s top law entrance exams.
Click this Video to learn more or speak to our experts now!




