India’s New Hope in Malaria Vaccine Development
CLAT Current Affairs 2026 Insight
GK & Current Affairs for CLAT | CLAT Current Affairs 2026
Powered by CLAT Gurukul – Best online coaching for CLAT
Why in News?
On July 23, 2025, the Indian Council of Medical Research (ICMR) announced a significant scientific breakthrough — the development of a candidate malaria vaccine named AdFalciVax. This vaccine is expected to provide over 90% protection against Plasmodium falciparum, the deadliest malaria parasite. The vaccine is now ready for human trials, and its animal testing phase has shown encouraging results. This development has the potential to become a game-changer in global public health and infectious disease control.
Given its national and global significance, and its link to healthcare policy, vaccine development, and disease eradication — this topic holds immense value for CLAT Current Affairs 2026.
Introduction
Malaria is one of the oldest and deadliest diseases known to humanity, killing nearly 400,000 people globally every year, especially in regions like sub-Saharan Africa and South Asia. While several malaria control measures have helped reduce its impact in recent years, an effective and affordable vaccine has remained elusive—until now.
India’s leading health research body, the Indian Council of Medical Research (ICMR), has developed AdFalciVax, a candidate vaccine that targets Plasmodium falciparum, which is responsible for a majority of severe and fatal malaria cases in India. If successful, this vaccine could revolutionize global efforts against malaria, much like polio vaccines transformed public health in the 20th century.
For CLAT aspirants, this topic offers intersections between health law, vaccine regulation, global health diplomacy, and intellectual property rights in pharmaceuticals—themes frequently tested in CLAT legal and general awareness sections.
Point-wise Summary
1. What is AdFalciVax?
- It is a candidate vaccine developed by ICMR that targets Plasmodium falciparum.
- Provides over 90% protection against infection.
- Developed in partnership with private companies for commercial testing and production.
- Currently undergoing pre-clinical (animal) testing; human trials will begin soon.
2. Why is this Vaccine Development Important?
- Plasmodium falciparum is the most dangerous malaria parasite, causing severe illness and death.
- Malaria causes symptoms such as:
- Fever, chills, vomiting, diarrhea
- Complications like seizures, lung failure, coma, and even death
- WHO reports indicate malaria remains a leading cause of mortality in many tropical nations, including India.
- India saw a decline in malaria deaths—from 1,707 in 2003 to just 83 in 2022—but vigilance is still needed to prevent resurgence.
3. Current Burden of Malaria in India and the World
- India: 30,000 reported malaria cases in 2022; death toll down to 83.
- Global burden: 400,000 deaths annually, mostly in Africa.
- Highly prevalent in countries like Nigeria, DRC, Tanzania, Mozambique, Uganda, and Burkina Faso.
- Malaria is a WHO-prioritized disease for eradication.
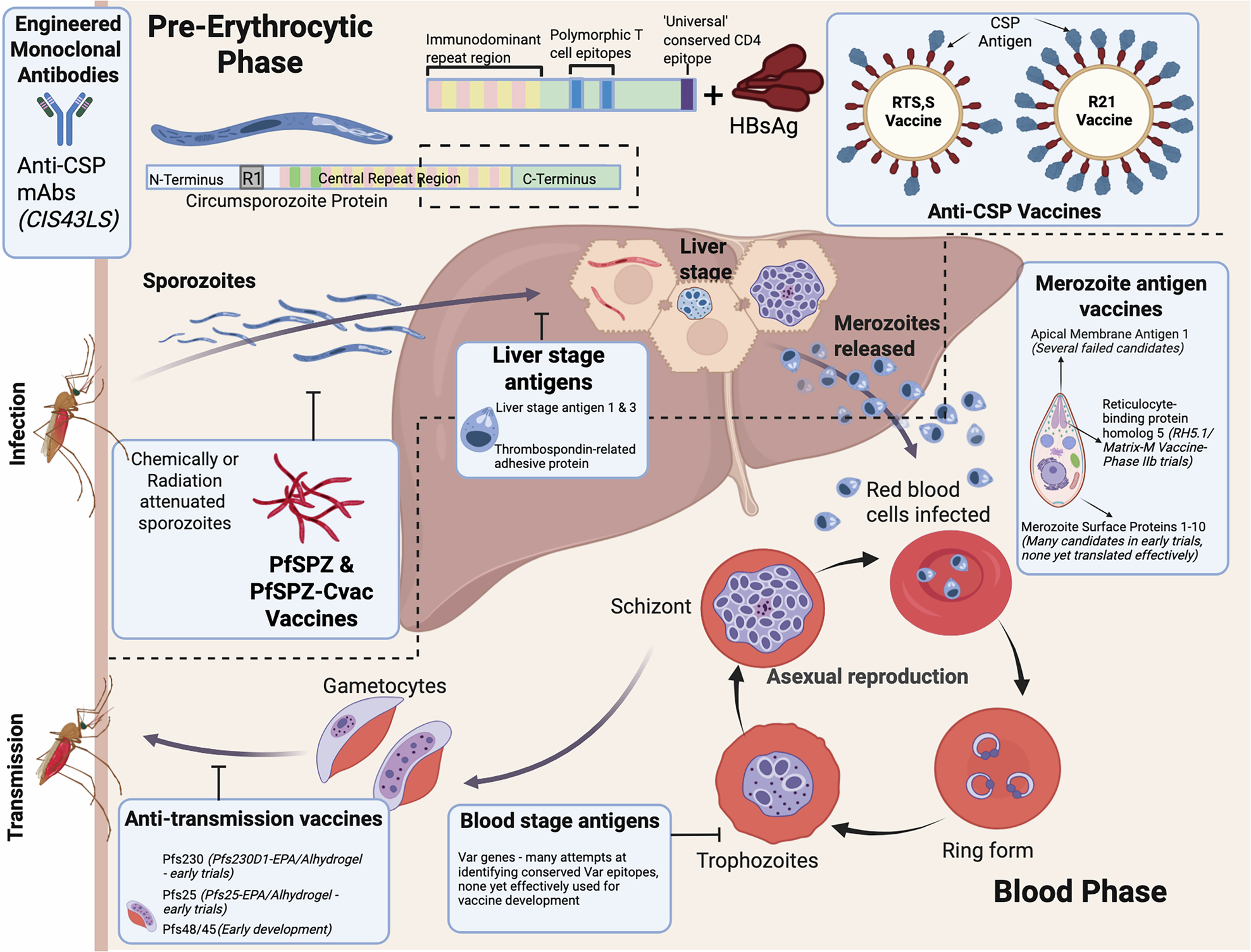
4. How Does AdFalciVax Work?
- It is an adenovirus-based recombinant vaccine:
- Uses harmless adenoviruses to deliver Plasmodium genes and generate an immune response.
- Targets two key components:
- Circumsporozoite protein (CSP) – helps parasite invade liver cells.
- PfGCS1 and Pf230 and Pf48/45 – proteins involved in parasite reproduction and blood-stage spread.
5. Difference from Existing Vaccines
- RTS,S (Mosquirix) and R21/Matrix M are WHO-approved but have limited efficacy (approx. 30–50%).
- AdFalciVax aims for greater protection (90%+).
- Uses alum as an adjuvant (immune booster), already used in many vaccines like hepatitis B and HPV.
- Shown to be safe in animals, causes no chronic inflammation.
6. Scientific and Regulatory Significance
- Provides a new mechanism to break malaria’s transmission cycle.
- If human trials are successful, India could become the first Asian country to roll out a high-efficacy malaria vaccine.
- May reshape the way vaccines are funded, tested, and distributed, especially in the Global South.
7. Challenges in Malaria Vaccine Development
- Malaria parasites mutate rapidly, making them difficult vaccine targets.
- Immune responses to malaria are complex and short-lived.
- Previous vaccines have had low efficacy and high costs.
- Human trials remain the biggest hurdle before approval and public rollout.
8. What is ICMR’s Role?
- ICMR is India’s apex body for biomedical research under the Ministry of Health.
- Has collaborated with industry partners to scale up AdFalciVax.
- The vaccine will now move to clinical testing, overseen by ICMR’s Regional Medical Research Centre in Dibrugarh.
9. Potential Legal and Policy Implications
- Vaccine regulation involves:
- Approval by Drugs Controller General of India (DCGI)
- Compliance with ICMR ethical guidelines
- Alignment with WHO’s emergency use listing (EUL)
- Intellectual property and licensing arrangements will play a major role in determining access and affordability.
10. Broader Impacts
- If successful, the vaccine could:
- Help eradicate malaria from South Asia.
- Save millions of lives, especially among vulnerable groups.
- Serve as a case study in vaccine diplomacy, much like India’s role in COVID-19 vaccine exports.
- Strengthen India’s position in global public health leadership.
Explanation of Key Terms
Term | Explanation |
AdFalciVax | A new candidate malaria vaccine developed by ICMR targeting Plasmodium falciparum. |
Plasmodium falciparum | Most deadly species of malaria parasite, responsible for majority of deaths. |
CSP (Circumsporozoite Protein) | A protein that helps the parasite invade liver cells, targeted by vaccines. |
Adjuvant | A substance added to vaccines to enhance immune response. Common adjuvants include alum. |
ICMR | Indian Council of Medical Research – the top government body for medical research in India. |
Adenovirus vector | A modified virus used to deliver vaccine components safely into human cells. |
RTS,S (Mosquirix) | WHO-approved malaria vaccine developed by GSK, with partial protection. |
PfGCS1 / Pf230 / Pf48/45 | Proteins from the malaria parasite involved in sexual stage development and transmission. |
Relevance for CLAT 2026 Aspirants
Legal Reasoning
- Potential passages based on:
- Vaccine testing and public safety
- Regulatory frameworks for drug approval
- Right to health and public interest
Current Affairs Section
- Important developments in:
- Science & tech policy
- Healthcare law
- Biotechnology and ethics
- Can be linked to constitutional principles like Article 21 (Right to Life).
Static Law + GK
- Involves bodies like:
- ICMR
- WHO
- DCGI
- Related to treaties and conventions like the TRIPS Agreement and Doha Declaration on Public Health.
Conclusion
The development of AdFalciVax by ICMR represents a critical leap forward in India’s fight against malaria, one of the world’s deadliest yet preventable diseases. With the potential to achieve more than 90% efficacy, the vaccine could revolutionize public health outcomes in malaria-endemic regions and strengthen India’s leadership in global health innovation.
For CLAT 2026 aspirants, this story is a textbook example of how scientific innovation intersects with law, policy, and ethics, making it a high-value topic for both the Current Affairs and Legal Reasoning sections of the exam.
This Blog is Powered by CLAT Gurukul — India’s Leading Law Entrance Prep Platform
At CLAT Gurukul, we believe in empowering future legal minds with the right blend of knowledge, strategy, and mentorship. This blog is a reflection of our commitment to quality content that not only helps aspirants stay updated but also sharpens their conceptual clarity.
Why CLAT Gurukul?
- Personalized Mentorship by Top Legal Educators
- Comprehensive Study Materials & Legal Updates
- Daily Practice Sets, Mocks & Performance Tracking
- Result-Oriented Strategy for CLAT, AILET, and CUET
Whether you’re reading this article to deepen your understanding or to stay ahead in your exam prep — you’re already one step closer with CLAT Gurukul by your side.
Join thousands of successful aspirants who trusted CLAT Gurukul and cracked India’s top law entrance exams.
Visit https://www.youtube.com/@CLATGurukul/shorts to learn more or speak to our experts now!
Note from CLAT Gurukul
At CLAT Gurukul, we are committed to providing free CLAT study material, including CLAT current affairs, legal reasoning practice sets, general knowledge updates, logical reasoning questions, English comprehension exercises, and more — all curated by top mentors.
Our blog section is regularly updated with high-quality CLAT content tailored to match the evolving pattern of the CLAT UG exam. Whether you’re looking for CLAT 2026 current affairs, CLAT legal reasoning passages, or mock practice sets, we have you covered.
We believe in open-access learning and will continue to publish free CLAT preparation resources to help serious aspirants succeed.
Explore more free content under categories like:
Best online coaching for CLAT, CLAT current affairs, CLAT GK updates, CLAT legal updates, CLAT logical reasoning, and CLAT English preparation.
For structured learning, daily mocks, and expert mentorship, visit https://www.youtube.com/@CLATGurukul/shorts — the Best CLAT Coaching in Patna and India’s most trusted platform for CLAT online coaching.




